News
 Podcast: Newly approved drug may slow progression of Alzheimer’s
Podcast: Newly approved drug may slow progression of Alzheimer’s
Washington University School of Medicine in St. Louis - Aug 4, 2023 Lilly drug slows Alzheimer’s by 35%, bolstering treatment approach
Reuters - May 8, 2023
 Conquering Alzheimer’s: a look at the therapies of the future
Conquering Alzheimer’s: a look at the therapies of the future
Nature - Apr 4, 2023
 Seeking Alzheimer’s clues from few who escape genetic fate
Seeking Alzheimer’s clues from few who escape genetic fate
AP News - March 16, 2023
 Lifetime Achievement Award
Lifetime Achievement Award
Clinical Trials on Alzheimer's Disease (CTAD) - March 14, 2023
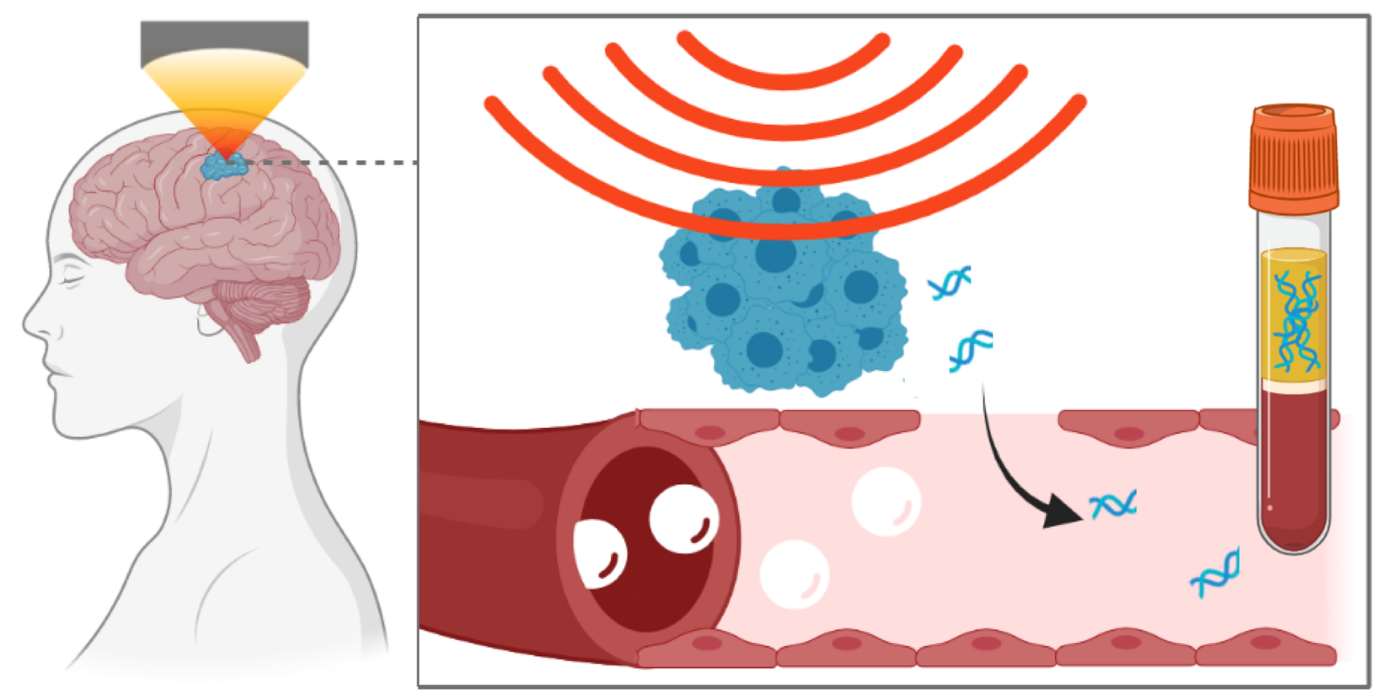 Focused ultrasound technique leads to release of neurodegenerative disorders biomarkers
Focused ultrasound technique leads to release of neurodegenerative disorders biomarkers
The Source, Washington University in St. Louis - Feb 3, 2023 Update on the DIAN-TU-001 Trial with E2814 and Lecanemab (Eisai Co., Ltd)
The statement below is in response to Eisai’s 06 January 2023 announcement regarding the FDA’s accelerated approval of lecanemab for...
FDA APPROVES LEQEMBI™ (LECANEMAB-IRMB) UNDER THE ACCELERATED APPROVAL PATHWAY FOR THE TREATMENT OF ALZHEIMER’S DISEASE
Eisai Co., Ltd. - Jan 06 2023Update on the DIAN-TU-002 Primary Prevention Trial
20 December 2022 Update on the DIAN-TU-002 Primary Prevention Trial The statement below is an update to the 15 November...



 WashU, Eisai form drug discovery collaboration
WashU, Eisai form drug discovery collaboration